Comparison of Different Membrane Filtration Systems for Recovering Active Phenolic Compounds from the European Spread Macroalga Sargassum muticum
Laurent Vandanjon1,2*, Carole Hersant1,3, Anaëlle Tanniou3, Fabienne Guerard3, Valérie Stiger-Pouvreau3
Affiliation
- 1University of Nantes, GEPEA, UMR CNRS 6144, IUML FR CNRS n°3473, F-44600 Saint-Nazaire
- 2University of South-Brittany, LBCM, EA 3884, IUEM, F-56000 Vannes, France
- 3University of Western Brittany, LEMAR, UMR 6539, IUEM, F-29280 Plouzané, France
Corresponding Author
Laurent Vandanjon, University of South Brittany, UFR SSI, F-56100 Lorient, France, Tel: +332.97.01.75.25, E-mail: laurent.vandanjon@univ-ubs.fr
Citation
Vandanjon, L., et al. Comparison of Different Membrane Filtration Systems for Recovering Active Phenolic Compounds from the European Spread Macroalga Sargassum muticum. (2017) J Marine Biol Aquacult 3(1): 1- 7.
Copy rights
© 2017 Vandanjon, L. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Proliferative macroalgae; Phenolic compounds; Sargassum muticum; Membrane processes
Abstract
Sargassum muticum is an invasive macroalga that uncontrollable widely grows on the European Atlantic coasts. This macroalga is particularly rich in phenolics (phlorotannins) presenting biological activities such as antioxidant, radical-scavenging, antibacterial, photoprotective properties, etc. Among the diversity of metabolites produced by the species, phenolic compounds could constitute one of the potential ways of valorization of Sargassum muticum.
For the needs of the study, algae were harvested along a latitudinal gradient in Norway, Ireland, France, Spain and Portugal.
After extraction, the highest phenolic contents and bioactivities were measured for the samples coming from Norway and Portugal. Consequently, both these countries have been retained in the present work.
The extracts are composed of a “pool” of phenolic compounds of molecular weight varying, according to the literature, from 126 Da (phloroglucinol) to 650 kDa. But the most common compounds are comprised between 10 and 100 kDa. With the aim of concentrating and eventually fractionating (i.e. isolating the most active fractions) the phenolic extracts of Sargassum muticum, a few membranes of Molecular Weight Cut-Off 10 kDa were chosen. These membranes were tested by using different technologies: ultrafiltration, centrifugal tube and dialysis.
Results show that there is no significant activity in the permeates signifying that the retention rate is about 100 % for the active fractions. Concerning the retentates, results show clearly an increase of the total phenolic contents as well as antioxidant and radical scavenging activities. The best performances are obtained with the centrifugal tubes using 10 kDa membranes.
Introduction
Marine macroalgae spreading along the intertidal rocky areas, as invasive species, produce high biomass[1] and can have an economic importance[2]. Among the diversity which occur in marine environment, i.e. 1726 species of Ulvophyceae, 6758 Florideophyceae and Bangiophyceae and 2048 Phaeophyceae[3], Brittany (France) is considered as a hot-spot region concerning macroalgae with nearly 700 species of macroalgae currently listed on the Breton littoral[4].
The present study is focused on Sargassum muticum, (Yendo) Fensholt (1955). This brown macroalga was introduced accidentally on the European coasts in the 1970s with oyster splats imported from Japan[5]. From its introduction point (United Kingdom), the alga spread along the Atlantic coast and enlarged its repartition. It is now observed from Norway to Morocco[6-9], and also on the French Mediterranean coasts[10]. Its proliferation is due to intrinsic characteristics such as a great tolerance to the variations of temperature and salinity, fast growth, mode of reproduction and dispersal, as well as the favourable conditions met on the European coasts. This proliferation cannot be controlled and it hampers certain human activities in summer period[9]. However, this abundant and under exploited marine living resource contains a lot of bioactive substances whose applications are numerous in food industry, cosmetics or pharmaceutics[11-23]. As demonstrated in the majority of the previously cited references, Sargassum muticum is particularly rich in phenolic compounds (phlorotannins) presenting biological activities such as antioxidant, radical-scavenging, antibacterial, photoprotection, etc. Industrialists are very interested by natural antioxidants in food industry because of the restricted use of synthetic anti-oxidants suspected to have toxic effects[24,25]. So, the extraction of phenolic compounds constitutes one of the main potential ways of valorization of Sargassum muticum.
Most often, phenolic extracts are too diluted to be used directly in the industry and so, they need to be concentrated. But the elimination of water by evaporation may denaturate the heat-sensitive phlorotannins. So, membrane processes can be used because they allow concentrating a sample at low or ambient temperatures. Some studies have been reported on the use of ultrafiltration to concentrate phenolic compounds from terrestrial plants[26-31], on the use of nanofiltration to produce phenolic rich fractions from artichoke[32] or orange press liquors[33], even on the use of electrodialysis applied to phenolics from tea[34]. All these articles cited as examples and others listed in the literature survey of Bazinet[35] show that membrane techniques are efficient for the separation of phenolics but very few studies have been published on marine resources such as macroalgae, except the use of ultrafiltration and/or dialysis for fractionating phlorotannins from brown algae[14,36,37].
In this study, with the aim of fractionating the phenolic extracts of Sargassum muticum (i.e. isolating the fractions with the highest antioxidant activity), a few membranes of Molecular Weight Cut-Off (MWCO) 10 kDa were chosen. Indeed, the extracts from Sargassum are composed of a “pool” of phenolic compounds of Molecular Weight (MW) varying from 126 Da (phloroglucinol) to 650 kDa (polymers) with most of the common compounds comprised between 10 and 100 kDa[38]. It is well known that the molecular size has a role in the bioactivity level of polyphenolics, although some authors claim that all the MW are active after ultrafiltration of extracts from Sargassum with membranes 10kDa[39,40]. So, in this study, different membrane based separations commonly used at the laboratory scale, i.e., ultrafiltration, membrane centrifugal tube and dialysis, were tested in order to validate the feasibility of the separation of active phlorotannins from Sargassum muticum.
Materials and Methods
Biological Material and Collection
Sargassum muticum is a pseudo-perennial species which settled in rocky shores in intertidal and subtidal zones in Brittany[9]. The perennial part is the main axis which persists all along the year. The annual parts are the flexible laterals visible from spring to summer.
For the needs of the study, individuals of Sargassum muticum were harvested along a latitudinal gradient from Norway, Ireland, France, Spain to Portugal on three different sites in each country (Table 1).
Table 1: Sites of Collection of Samples of Sargassum muticum.
| Country | Region | Site | Code | Latitude | Longitude | Hydrodynamism |
|---|---|---|---|---|---|---|
| Norway (N) | Øygarden | Solberg | N1 | 36°35’N | 4°47’W | Semi-exposed |
| Øygarden | Kraekjebaebaerholmen | N2 | 36°35’N | 4°50’W | Semi-exposed | |
| Øygarden | SauØyna | N3 | 36°35’N | 4°51’W | Semi-exposed | |
| Ireland (I) | Connaught | Clifden | I1 | 53°40’N | 10°11’W | Exposed |
| Munster | Spanish point | I2 | 52°85’N | 9°44’W | Semi-exposed | |
| Munster | MiltownMalbay | I3 | 52°84’N | 9°44’W | Semi-exposed | |
| France (F) | Brittany | Grand Dellec | F1 | 48°21’N | 4°34’W | Exposed |
| Brittany | Le Minou | F2 | 48°20’N | 4°36’W | Exposed | |
| Brittany | Pointe du diable | F3 | 48°21’N | 4°33’W | Exposed | |
| Spain (S) | Galicia | Museo del Mar | S1 | 42°13N | 8°46’W | Semi-exposed |
| Galicia | Caboes tai | S2 | 42°11’N | 8°48’W | Exposed | |
| Galicia | Caboudra | S3 | 42°20’N | 8°49’W | Exposed | |
| Portugal (P) | Algarve | Queimado | P1 | 37°49N | 8°47W | Semi-exposed |
| Algarve | Almogave | P2 | 37°39’N | 8°48’W | Semi-exposed | |
| Algarve | Zambujeira | P3 | 37°33’N | 8°47W | Semi-exposed |
The collection of samples was done in April-May at the same level of physiological development (end of the growth phase). Only the apical part was collected to minimize the environmental impact. After sampling, epiphytes were removed and the thalli were rinsed with fresh water before freeze-drying and powder grinding[41].
Extraction and Purification of Phenolic Compounds
Phenolic compounds were extracted from freeze-dried algal powder by a mixture of acetone: water (50: 50) at 40°C for 2 hours in the dark. The extract was then centrifuged, filtered and vacuum evaporated before adjusting the volume of the raw extract to 50 mL with distilled water[16].
Analysis of the Total Phenolic Content and Activity Tests of the Extracts/Fractions
The Folin-Ciocalteu colorimetric method modified for microplates by Sanoner et al[42] was used to measure the total phenolic content of extracts/fractions. Two types of activities have been evaluated:
Radical-scavenging activity with the DPPH test on microplates by following the method described by Fukumoto and Mazza[43]. The results were expressed by the IC50 index, which is the concentration (mg/mL) of phenolic compounds necessary to inhibit 50% of the reducing reaction of the DPPH. So, a low value of IC50 corresponds to a high radical scavenging activity.
Antioxidant activity with the β-carotene bleaching test on microplates was carried out following the methodology described by Kaur and Kapoor[44]. Butyl Hydroxyanisole (BHA) was used as a positive control, whereas ethanol was the negative control. An Antioxidant Activity Coefficient (AAC) was then calculated as follows:
AAC = ((As (120) - Ac (120)) / (Ac (0) - Ac (120)) * 1000
As: Absorbance of the sample at 120 min
Ac: Absorbance of the control (ethanol) at 0 or 120 min
The AAC700 corresponds to the concentration of the extract (mg/mL) necessary to have an AAC of 700, which is the value obtained with the positive control as described by Le Lann et al[12]. So, a low value of AAC700 corresponds to a high antioxidant activity.
Analysis of the Extracts by Nuclear Magnetic Resonance (1H NMR)
With the aim of verifying the presence of phenolic compounds in the crude and purified extracts, we analysed our extracts/fractions with 1H NMR. Then, the extracts/fractions were mixed with deuterized methanol (MeOD) at room temperature and were analysed with a spectrometer BRUKER DRX 400 using the standard pulse sequences available in the Bruker software (Bruker, Wissembourg, France).
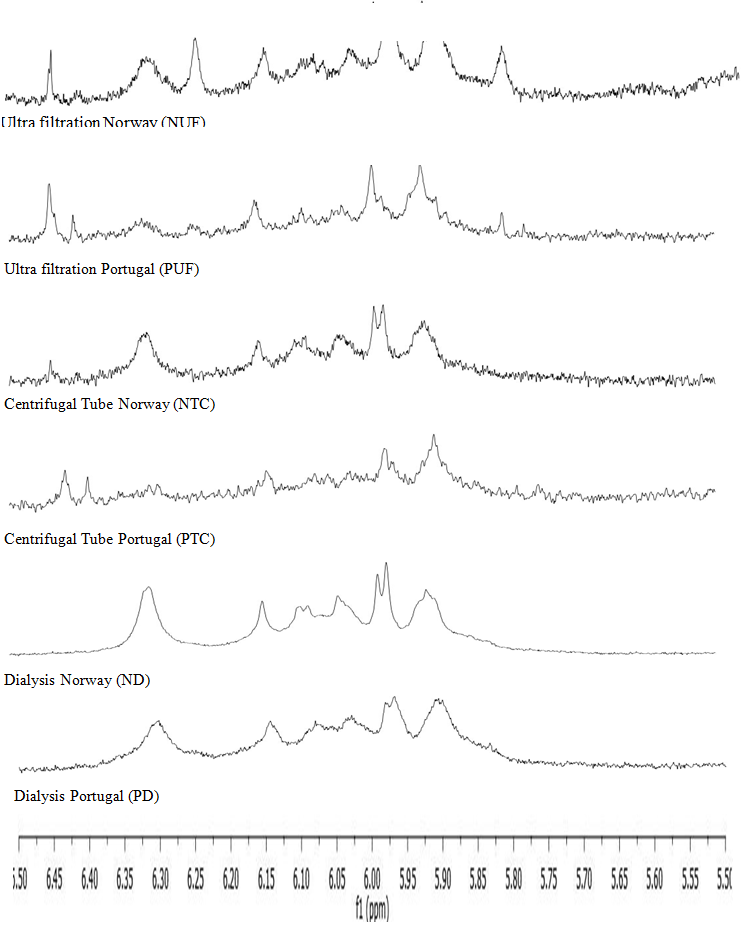
Chemical shifts were expressed in ppm. NMR profiles unambiguously indicate the presence of phenolic compounds within the extracts, with peaks between 5.5 and 6.5 ppm.
Molecular Weight Separation of Phenolic Compounds
In order to concentrate under mild conditions the phenolic extracts, membrane filtration systems were chosen. Three different processes (Ultrafiltration, Membrane centrifugal tubes and Dialysis) often used at the laboratory scale were selected. All the experiments were carried out by using membranes of MWCO 10 kDa that may normally retain the most common phenolic compounds whose MW is comprised between 10 kDa and 100 kDa.
Ultrafiltration
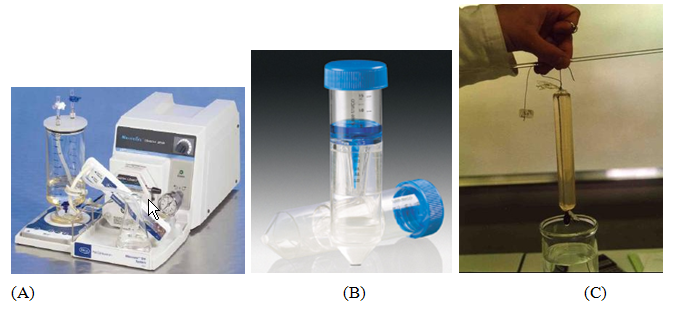
The ultrafiltration system was a Minimate Tangential Flow Filtration capsule (PALL) of 50 cm2 in polyethersulfone (Figure 1A). The filtration was carried out at a pressure of 2.105 Pa and a temperature of 20°C with membranes of MWCO 10 kDa.
Membrane Centrifugal Tubes
Membrane centrifugal tubes (Figure 1B) equipped with membranes in regenerated cellulose (Hydrosart) of surface area 3.9 cm2 and MWCO 10 kDa were used at a centrifugal acceleration of 3000 g and a temperature of 15°C.
Figure 1: Different membrane filtration systems: (A) Ultrafiltration, (B) Membrane centrifugal tubes, (C) Dialysis.
Dialysis
Membranes in cellulose ester of MWCO 10 kDa constituted the dialysis chamber filled with the extracts. The dialysis bag was put for 28 hours in distilled water (renewed 3 times) (Figure 1C).
Results
Phenolic Content of the Crude Extracts
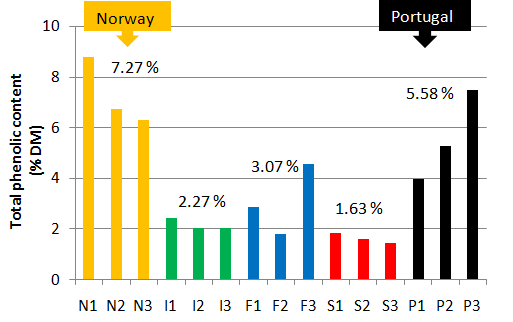
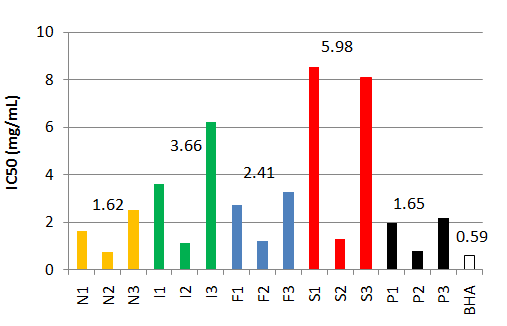
The total phenolic contents (expressed in % of Dry Matter DM) of the crude extracts have been evaluated for the 3 sites in each country[17]. It can be observed on Figure 2 that the mean values are the highest for Norway (7.27 %) and Portugal (5.58 %). These results are confirmed by the measurement of the radical-scavenging activity (Figure 3) which is on average the lowest for Portugal (1.65 mg/mL) and Norway (1.62 mg/mL). The best values IC50 = 0.791 mg/L for the site 2 in Portugal (P2) and IC50 = 0.738 mg/L for the site 2 in Norway (N2) are very close to the positive control (0.590 mg/mL)).
Figure 2: Total phenolic content (expressed in % of Dry Matter DM) in the raw extracts (N: Norway, I: Ireland, F: France, S: Spain, P: Portugal) Numbers 1,2,3 correspond to the three sites sampled by country.
Figure 3: Radical-scavenging activities in the crude extracts (N: Norway, I: Ireland, F: France, S: Spain, P: Portugal, BHA: Butyl Hydroxyanisole) Numbers 1,2,3 correspond to the three sites sampled by country.
For that reason, the rest of this study will be focused on the phenolic extracts from site 1 Norway (N1) and site 3 Portugal (P3).
Fractionation of Phenolic Compounds by Membranes The raw extracts have been concentrated by ultrafiltration, centrifugal tube and dialysis, implemented with membranes of MWCO 10 kDa.
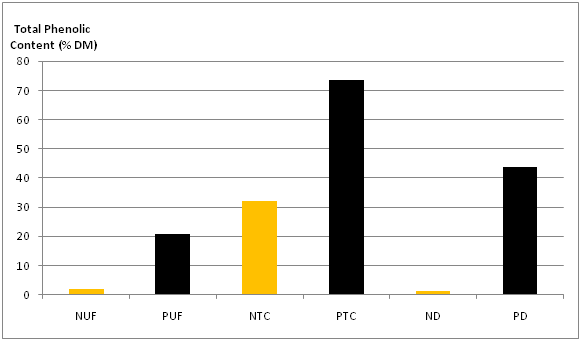
Only negligible amounts of phlorotannins were detected in the permeates, whereas some retentates were highly enriched in phenolic compounds, especially after concentration with centrifugal tube. In this latter case, it has been reached a maximum total phenolic concentration in the retentate of 73.5% for Portugal (Figure 4).
Figure 4: Total phenolic content in the retentates (N: Norway, P: Portugal, UF: Ultrafiltration, TC: Centrifugal Tube, D: Dialysis).
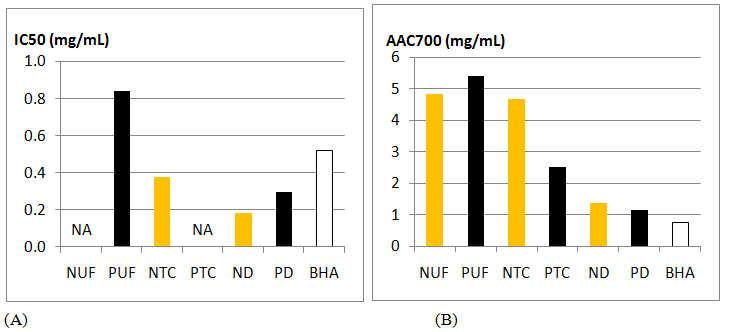
Concerning the radical-scavenging and the antioxidant activities, the most active retentates are obtained after dialysis with excellent IC50 and AAC700 close to positive control BHA (Figure 5A and Figure 5B) for Norway and Portugal.
Figure 5: Activities of the retentates obtained in extracts issued from populations of Sargassum muticum collected in Norway and Portugal. (A) Radical scavenging activity, (B) β-carotene bleaching activity, (N: Norway, P: Portugal, UF: Ultrafiltration, TC: Centrifugal Tube, D: Dialysis, BHA: Butyl Hydroxyanisole)
The analysis of the retentates by 1H-NMR has shown that the three processes (ultrafiltration, centrifugal tube, dialysis) are efficient to concentrate phlorotannins. But the most accurate peaks are obtained after dialysis (Figure 6) meaning a better purity of the concentrated extract.
Figure 6: 1H-NMR analysis of the retentates with enlargement of the spectra in the aromatic area between 5.5 and 6.5 ppm (phenolic compounds).
Discussion
The industrial valorization of the invasive brown algae has already been studied[45] but there is still much to do. In particular, phenolic compounds (phlorotannins) from brown macroalgae are not industrially exploited and commercialized for their antioxidant properties. So, it is important for future industrial applications to determine the phenolic contents in brown macroalgae Sargassum muticum (taking into account the variability of the resource), to measure the activities according to the molecular size of the phlorotannins and to evaluate the feasibility of the concentration by membrane technologies.
Populations of Sargassum muticum collected in Norway and Portugal have higher total phenolic contents and higher radical-scavenging activities than populations from Ireland, France or Spain. This is in accordance with previous studies showing that the biochemical composition (proteins, lipids, carbohydrates) of Sargassum muticum is linked to the geographical site and to the hydrodynamic conditions[41]. It can be supposed that the severe environmental conditions at the extremities of the latitudinal gradient promote the production of phlorotannins, considered as defensive compounds against the UV or the variations of temperature. However, for the countries (France, Ireland, Spain) where phenolic content is not enough, Sargassum muticum could be cultivated under stressful conditions (metabolic orientation).
The most common phenolic compounds have a MW between 10 and 100 kDa[46], that seems to be the case with Sargassum muticum from Norway and Portugal while no phlorotannins were detected in the permeates of the membranes 10 kDa. The phenolic compounds of low MW (less than 2000 Da) are very rare in temperate Sargassaceae[14] whereas these small compounds are very abundant in tropical Sargassaceae (South Pacific)[47].
The NMR spectra of the retentates are quite similar for ultrafiltration, centrifugal tube and electrodialysis, which suggest the presence of the same type of phenolic compounds. But a further study using HPLC for determining the size repartition of phlorotannins would be necessary, as it was demonstrated in another study on fish protein hydrolysates[48]. Then, the concentration by tangential flow filtration will be possible at the industrial scale, either by ultrafiltration or nanofiltration as it was proved to be interesting in the Magnoliophyta, mate[49]. And finally, fractionation will also be possible on the condition of using membranes of MWCO higher than 10 kDa.
Conclusion
This study has shown that the concentration by membranes of the phlorotannins from Sargassum muticum is possible. In this case, the main advantage of the membranes is to recover phenolic compounds without denaturating the other molecules. It sees an opportunity to develop an algorefinery process consisting in the recovery of the main target molecules (phlorotannins) and the potential secondary target soluble compounds (proteins, polysaccharides, pigments, alginates, etc), as well as the solid fraction. To reach this objective, the combination of membranes with mild extracting technologies, such as enzymatic hydrolysis for instance, is a promising route[50].
Acknowledge:
Results of this study are included within the PhD work carried out by A. Tanniou. The work was supported by the Interreg IVB project Biotecmar for the sampling along Atlantic coast. Moreover, analysis were supported by two projects: the Région Pays de La Loire and the MSH AngeGuépin, France (COSELMAR project) and the Era-net/seas era/ANR project INVASIVES.
References
- 1. Cabioc’h, J., Floc’h, J.Y., Le Toquin, A., et. al. Guide des algues des mers d’Europe. (2006) DelachauxetNiestlé S272
- 2. Bourgougnon, N., Stiger-Pouvreau, V. Chapter 4: Chemodiversity and bioactivity within red and brown macroalgae along the french coasts. (2011) In Handbook of Marine Macroalgae (ed. S.K. Kim) S58-S105.
- 3. Guiry, M.D., Guiry, G.M. AlgaeBase. (2016) World-wide electronic publication, National University of Ireland, Galway.
- 4. Dizerbo, A.H., Herpé, E. Liste et répartition des algues marines des côtes françaises de la Manche et de l’Atlantique, Iles Normandes incluses. (2007) Ed. Anaximandre, Landernau 1: S315.
- 5. Critchley, A. T., Farnham, W. F., Yoshida, T., et. al. A bibliography of the invasive alga Sargassum muticum. (1990) Botanica Marina 33(6): S551-S562.
- 6. Plouguerné, E., Le Lann, K., Connan, S., Jechoux, G., Deslandes, E., Stiger-Pouvreau, V. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalgaSargassummuticum along the coast of Western Brittany. (2006)Aquatic Botany 85: 334-337.
- 7. Cacabelos, E., Olabarria, C., Viejo, R. M., et.al. Invasion of Sargassum muticum in intertidal rockpools: patterns along the Atlantic Iberian Peninsula. (2013) Marine Environmental Research 90: S18-S26.
- 8. Sabour, B., Reani, A., El Magouri, H., et.al. Sargassum muticum (Yendo) Fensholt (Fucales, Phaeophyta) in Morocco, an invasive marine species new to the Atlantic coast of Africa. (2013) Aquatic Invasions 8(1): S97–S102.
- 9. Stiger-Pouvreau, V., Thouzeau, G. Marine species introduced on the French channel-Atlantic coasts: A review of main biological invasions and impacts. (2015) Open J Ecol 5: S227–S257.
- 10. Boudouresque, C. F., Verlaque, M. Biological pollution in the Mediterranean sea: invasive versus introduced macrophytes. (2002) Marine Pollution Bulletin 44: S32-S38.
- 11. Swanson, A.K., Druehl, L.D. Induction, exudation and UV protective role of kelp phlorotannins. (2002) Aquatic Botany 73(3): S241-S253.
- 12. Le Lann, K., Jégou, C., Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: comparison of the frondose Sargassummuticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. (2008) Phycol Res 56(4): S238-S245.
- 13. Plouguerné, E., Georgantea, P., Ioannou, E., et.al. Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. (2010) Mar Biotechnol 12(1): S52-S61.
- 14. Le Lann, K., Connan, S., Stiger-Pouvreau, V. Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: native versus introduced species. (2012) Marine Env Res 80: S1-S11.
- 15. González-López, N., Moure, A., Domínguez, H. Hydrothermal fractionation of Sargassum muticum biomass. (2012) J Appl Phycol 24(6): S1569–S1578.
- 16. Tanniou, A., Serrano Léon, E., Vandanjon, L., et.al. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. (2013) Talanta 104: S44-S52.
- 17. Tanniou, A., Vandanjon, L., Incera, M., et.al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. (2014) J Appl Phycol 26(2): S1215-S1230.
- 18. Stiger-Pouvreau, V., Jégou, C., Cérantola, S., et.al. Phlorotannins in Sargassaceae species from Brittany (France): interesting molecules for ecophysiological and valorisation purposes. (2014) Adv Botanical Res 71: S379-S411.
- 19. Conde, E., Moure, A., Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. (2015) J Appl Phycol 27(2): S957–S964.
- 20. Balboa, E.M., Moure, A., Domínguez, H. Valorization of Sargassum muticum Biomass According to the Biorefinery Concept. (2015) Mar Drugs 13(6): S3745-S3760.
- 21. Balboa, E.M., Gallego-Fábrega, C., Moure, A., et.al Study of the seasonal variation on proximate composition of oven-dried Sargassummuticum biomass collected in Vigo Ria, Spain. (2016) J. Appl. Phycol 28(3): S1943-S1953.
- 22. Montero, L., Sánchez-Camargo, A.P., García-Canas, V., et.al. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. (2016) J Chromatogr A 1428: S115–S125.
- 23. Milledge, J.J., Nielsen, B.V., Bailey, D. High-value products from macroalgae: the potential uses of the invasive brown seaweed, Sargassum muticum. (2016) Rev Environ Sci/Biotechnol 15(1): S67-S88.
- 24. Ito, N., Nishi, K., Nakajima, M., et. al. Effects of alpha-galactosidase digestion on lectin staining in human pancreas. (1988) Histochemistry 89(2): S121-S128.
- 25. Bandoniené, D., Pukalskas, A., Venskutonis, P.R., et. al. Preliminary screening of antioxidant activity of some plant extracts in rapeseed oil. (2000) Food Res Internat 33(9): S785-S791.
- 26. Nawaz, H., Shi, J., Mittal, G.S., et. al. Extraction of polyphenols from grape seeds and concentration by ultrafiltration (2006) Sep Purification Technol 48(2): S176-S181.
- 27. Gökmen, V., Acar, J., Kahraman, N. Influence of conventional clarification and ultrafiltration on the phenolic composition of golden delicious apple juice. (2003) J Food Quality 26(3): S257-S266.
- 28. D’Alvise, N., Lesueur-Lambert, C., Fertin, B., et. al. Removal of polyphenols and recovery of proteins from protein concentrate by UF and adsorbent resin separations. (2000) Separation sci Technol 35(15): S2453-S2472.
- 29. Decloux, M., Tatoud, L., Mersad, A. Removal of colorants and polysaccharides from raw cane sugar remelts by Ultrafiltration. (2000) Zuckerindustrie 125(2): S106-S113.
- 30. El Abbassi, A., Kiai, H., Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. (2012) Food Chem 132(1): S406-S412.
- 31. Susanto, H., Feng, Y., Ulbricht, M. Fouling behavior of aqueous solutions of polyphenolic compounds during ultrafiltration. (2009) J Food Eng 91(2): S333-S340.
- 32. Cassano, A., Cabri, W., Mombelli, G., et. al. Recovery of bioactive compounds from artichoke brines by nanofiltration. (2016) Food Bioproducts Proc 98: S257-S265.
- 33. Conidi, C., Cassano, A., Drioli, E. Recovery of phenolic compounds from orange press liquor by nanofiltration. (2012) Food Bioproducts Proc 90(4): S867-S874.
- 34. Labbe, D., Araya Farias, M., Tremblay, A., et. al. Electromigration feasibility of green tea catechins. (2005) J Membr Sci 254(1-2): S101-S109.
- 35. Bazinet, L. Antioxidant recovery by membranes. (2015) Encyclopedia of membranes, Springer Verlag Berlin Heidelberg S1-S5.
- 36. Ar Gall, E., Lelchat, F., Hupel, M., et. al. Extraction and purification of phlorotannins from brown algae. (2015) Methods in Mol Biol 1308: S131-S143.
- 37. Breton, F., Cérantola, S., Ar Gall, E. Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). (2011) J Exp Mar Biol Ecol 399(2): S167-S172.
- 38. Targett, N., Arnold, T. Effects of secondary metabolites on digestion in marine herbivores. (2001) In: McClintock J. B., Baker B.J. (eds) Marine Chem Ecol S391-S411.
- 39. Lu, H.Y., Liu, Y., Shao, H.Y., et. al. Antioxidant and antiproliferative effects of phlorotannins from Sargassum mcclurei. (2012) Food Sci 2012-23.
- 40. Gu, L.X., Pan, C.Y., Zhao, S.L., et. al. Study of antidepressant effect of phlorotannins with different molecular weight extracted from Sargassum fusiforme. (2015) Lishizen Medicine and Materia Medica Res 2015-02.
- 41. Tanniou, A., Vandanjon, L., Goncalves, O., Kervarec, N., Stiger-Pouvreau, V. Rapid geographical differentiation of the European spread macroalgaSargassummuticum using HRMAS-NMR and Fourier-Transform Infrared spectroscopy. (2015)Talanta 132: 451-456.
- 42. Sanoner, P., Guyot, S., Marnet, N., et. al. Polyphenol profiles of French cider apple varieties. (1999) J Agric Food Chem 47(12): S4847-S4853.
- 43. Fukumoto, L.R., Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. (2000) J Agric Food Chem 48(8): 3597-3604.
- 44. Kaur, C., Kapoor, H.C. Antioxidant activity and total phenolic content of some Asian vegetables. (2002) Intern J Food Sci Technol 37(2): S153-S161.
- 45. Zubia, M. La valorisation industrielle des algues brunes invasives (fucales) de Polynésie française: étude prospective pour lutter contre leur prolifération et contribuer à la gestion durable de l’environnement récifal. (2003) PhD thesis, Université de Polynésiefrançaise.
- 46. Boettcher, A., Targett, N. Role of polyphenolic molecular size in reduction of assimilation efficiency in Xiphistermucosus. (1993) Ecology 74(3): S891-S903.
- 47. Le Lann K., Ferret C., VanMee E., et. al. Total phenolic, size fractionated phenolics and fucoxanthin content of tropical Sargassaceae from the South Pacific Ocean. (2012) Phycol Res 60(1): S37-S50.
- 48. Chabeaud, A., Vandanjon, L., Bourseau, P., et. al. Performances of ultrafiltration membranes for fractionating a fish protein hydrolysate. (2009) Separat Purification Technol 66(3): S463-S471.
- 49. Murakami, A., Amboni, R., Prudencio, E., et. al. Concentration of phenolic compounds in aqueous mate extract through nanofiltration. (2011) Food Sci Technol 44(10): S2211-S2216.
- 50. Vandanjon, L., Vallet, L., Le Glatin, T., et. al. Valorization of the macroalgae Sargassum muticum by enzymatic hydrolysis, interest of surfactants to improve the extraction of phlorotannins and polysaccharides. (2016) J Marine Biology Aquaculture, Ommega Publishers 2(1): S1-S7.